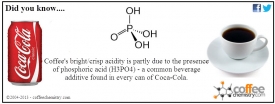
Well as it turns out there are a number of reasons why Coke purposely adds phosphoric acid to its products - with perhaps the most important being its ability to act as a preservative.
By adding phosphoric acid (H3PO4) manufacturers are able to significantly lower the pH of the beverage thereby preventing the growth of microorganisms that would otherwise spoil the beverage while in the can. Although the pH varies between the various versions of Coke (eg. Coke Classic, Coke Zero, etc) most beverages range in the 2-3 pH range - making it very much acidic.
Coincidentally phosphoric acid is also found in coffee and provides for the unique bright/crisp acidity that we commonly find in East African coffees. Anyone that has ever cupped a Brazilian alongside a Kenyan coffee knows the difference in acidity is 'night and day' - that my friend, is phosphoric acid!
Although there is still much research to be conducted studies have shown that coffee contains a mixture of about thirty acids in solution. As such researchers have suggested that phosphoric acid may play a critical role in the development of acidity - perhaps even more so than the other acids present in the cup.