According to researchers, chlorogenic acid and the formation of brown-colored melanoidins are most responsible for coffee's tremendous "antioxidant capacity".
Although these compounds exist in green coffee, the vast majority are formed during the roasting process. But not all coffee provides the same level of antioxidant capacity, as shown in Figure 1. The coffee that have been decaffeinated/mild or rendered "soluble" have significantly less antioxidant capacity than regular drip coffee.
Figure 1: Antioxidant Capacity by Coffee Type
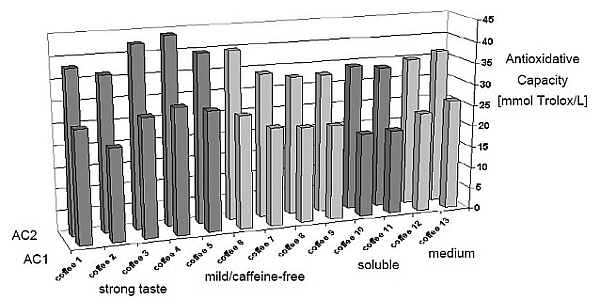
Although the terms "strong taste" and "medium' were used in the study, our guess is that this was simply the author's expression of the coffee's concentration and not so much an expression of roast level (i.e. strong meaning a coffee with a darker roast).
So, why would decaf and soluble coffee have lower antioxidant capacity? Well, turns out that the additional processing, e.g. steaming, spray drying, etc. destroys antioxidants contained in coffee and render is less beneficial.
What was interesting to see in the study is, that when compared to data showing roasting temperatures, those coffees roasted to 220°C (428°F) showed the greatest concentration of antioxidants than those roasted to 217°C (422.6°F) and 228°C (442.4°F), suggesting an optimal roasting profile. Interesting - unroasted coffee was also compared as shown in Figure 2.
Figure 2: Antioxidant Capacity by Roasting Bean Temperature
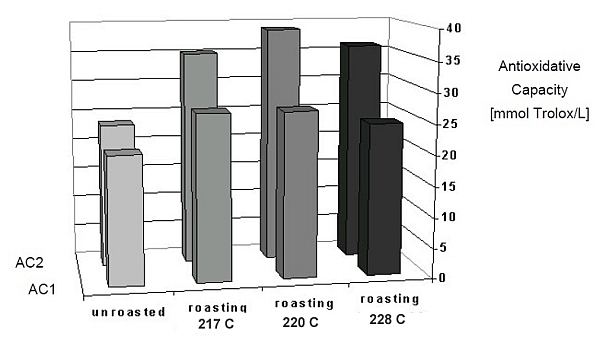
So there you go, a basic discussion of coffee antioxidants. But we're sure many of you now may have more questions than answers, so feel free to leave your comments/questions below.
Notes:
- AC1 and AC2 are simply two types of antioxidants tested for using TEAC
- No details were provided in the study about roasting specs

