Chlorogenic Acid in coffee
Sensorially quinic and caffeic acid have been associated with the increased levels of astringency, bitterness and body commonly seen in darker roasting styles.
Selected composition for raw coffee (%): 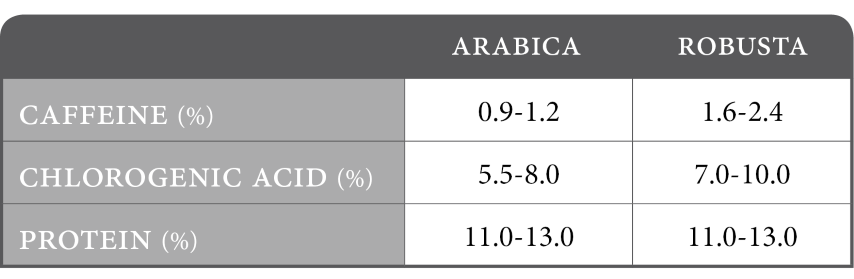
Coffee has by far the largest concentration of chlorogenic acid of any species in the plant kingdom, accounting for 6-7% d.b. in Arabica and up to 10% d.b. in Robusta.
CGA production in the plant is initiated by several factors including changes in environmental conditions, plant stress and pest infestation. It is no surprise then, that robusta, while grown in harsher conditions contains almost twice the concentration of CGA than arabica.
CGA production also closely parallels with that of caffeine, such that, as CGA concentrations increase so does the concentration of caffeine.

Although the term chlorogenic acid is used to identify a single compound, in reality, there are over a dozen isomers each with different sensorial characteristics. Typically it is the 3-CGA isomer who predominates in coffee with mono, di and feruloylquinic acid in varying concentrations.
Research has suggested that the "di-CGA" form of the acid may be responsible to the bitter/metallic taste notes found in certain coffees. This may very well be true for coffees like robusta which contain a significant higher concentrations of di-CGA and harsher taste profile.
 |
| 3,4-dicaffeoylquinic acid (di-CQA) |
Latest research has confirmed that coffee contains high concentrations of antioxidants in the range of 200 to 550mg per cup (6oz) - a level far exceeding that of green tea. But although both coffee and tea contain high levels of antioxidants, coffee tends to contain higher levels of the simple phenolics whereas tea larger levels of catechins.
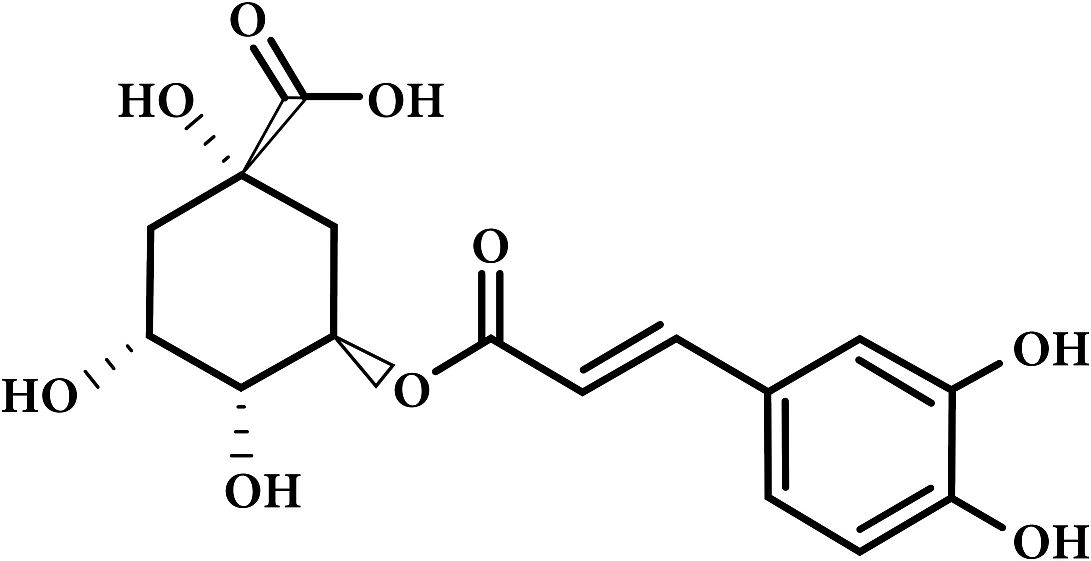
Chlorogenic Acid
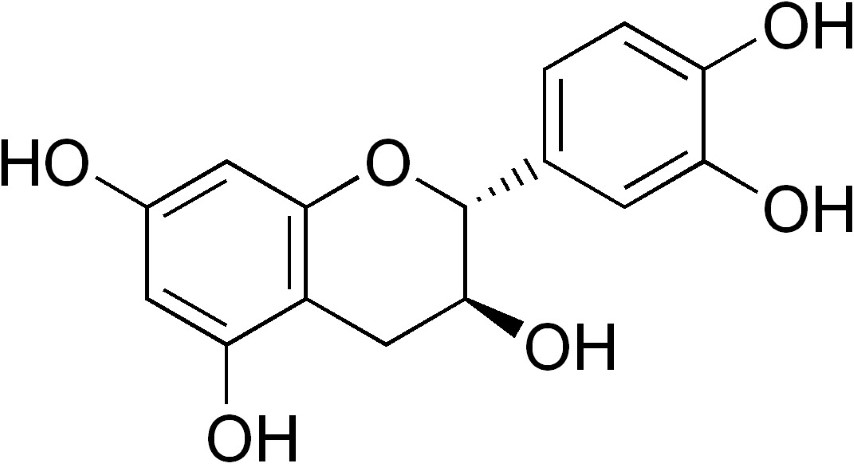
Catechin